Finding Answers in Tears
Another of my final Arkansan stories was a feature of a small startup doing some big, revolutionary work. A team of four at Ascendant Dx in Springdale, AR is working to completely change the way breast cancer screenings happen. They've developed a test that detects breast cancer biomarkers in human tears in under 30 minutes. It will cost $99.
Right now, mammograms are all we've got. They're only about 50% accurate, they're expensive and annoying. Plus, most of the world doesn't even have access to them. Without mammograms, those women generally only find out they have breast cancer once it's advanced enough for them to have symptoms. As Omid Moghadam, the CEO of Ascendant pointed out to me, this is exactly the same way the first documented case of breast cancer was discovered 2,000 years ago in ancient Persia.
Ascendant Dx has been in the works in Arkansas for over 10 years now, and the team is now working towards full clinical trials and looking for funding. It's slow going as no one uses tears for any diagnostic testing, and the group is having to collect all their own samples manually. However, since blood is condensed to make tears in our systems, they claim this is a powerful substance with big potential to diagnose even more diseases in the future.
Arkansas Democrat-Gazette
Arkansas firm uses tears to diagnose breast cancer
A company in Springdale is working to revolutionize the detection of breast cancer with an unusual substance -- tears.
The team at Ascendant Dx has developed a test analyzing tears that could complement -- or even replace -- mammograms as the first step in looking for breast cancer.
Omid Moghadam, CEO and chairman, said that in middle- to lower-income countries without the funding or expertise to operate mammography on a large scale, Ascendant's Melody test would be a game changer.
"We think that biological tests like Melody would be perfect for that environment," he said of the company's product, which detects biomarkers found in tears to test for the disease within 30 minutes.
"It's inexpensive, accurate and someone with minimal training can run it." From there, he explained, patients who test positive can travel to larger medical centers for further testing and treatment.
Today, physicians are reimbursed through medical insurance between $250 and $350 per mammogram, while Ascendant's target retail price for Melody is $99.
In places like the U.S., which has $5 trillion worth of mammography equipment, there is also a lot of room for improvement, he said.
"In the U.S. we are still brute forcing breast cancer detection with mammography, because we had nothing until 30 years ago," he said.
"When you go from not having anything to something that is 50 percent accurate, that's what takes hold. And that's what we still have today: a not-so-good imaging technique that has 50 percent false positive, 50 percent false negative."
Dr. Suzanne Klimberg is a breast surgeon at the University of Arkansas for Medical Sciences' Rockefeller Cancer Institute who pioneered Ascendant's research. She remains the company's medical director and said early detection of breast cancer is the best thing until a prevention or cure is found.
"Access to care has been the biggest stumbling block," Klimberg said. "My dream has always been to have this on the market and, similar to the use of a pregnancy test, be able to screen yourself."
Moghadam referred to a study by Mei-Sing Ong and Kenneth Mandl in Health Affairs journal that found that of the $8 billion a year spent on mammography, $4 billion is wasted on false-positive diagnoses.
Among American women, breast cancer remains the second-most-diagnosed cancer and the second-most-common cause of cancer death.
The Ascendant story starts in 2006 with Klimberg at UAMS. She was inspired to work with human tears after seeing research on detecting the cancer in breast milk, realizing that the tissues that concentrate blood to produce both fluids are similar.
"It's an unusual fluid, but it has benefits," Moghadam said of tears.
"It's sterile, and it's a lot less complicated than blood," he said. "Blood has so many bits and pieces of cells and DNA that anytime you want to look for something, you have to filter lots of things out in order to find them. That becomes expensive."
The TRG Foundation in Little Rock funded Klimberg's initial research, and then it caught the attention of VIC Technology Venture Development, a Fayetteville venture creation company. That's where, Moghadam became involved, and in 2013, when it spun out of VIC, he became its CEO.
Dr. Steven Harms, a breast radiologist at the Breast Center in Fayetteville, said he was originally skeptical of the idea.
"At first I thought, 'This is very interesting, but I don't think it is going to work.' I was very skeptical, because it's almost too good to be true."
In the end, the results won him over. He now serves on the company's board, and the Breast Center has participated in the company's studies.
He recalled that two of his patients' traditional biopsies came back negative but their Ascendant's Melody tests came back positive. At first they dubbed those false positives, until the patients returned a few months later with cancerous biopsies.
He said someday, a test like Melody has the potential to replace mammograms as the first step in the process, though there will always need to be an imaging method like mammography to locate where the cancer is.
"Wouldn't it be nice if we could have people come in with a high probability of having cancer in the beginning and concentrate our resources on finding that cancer instead of just screening everybody?" he said.
Plus, he pointed out, only half of American women above 40 actually get mammograms for reasons like cost, discomfort and geographic access -- things that Melody could overcome.
Ascendant has collected around 700 samples from three validation studies so far. The next step is a clinical trial with 1,000 more subjects to gain regulatory approval in the U.S. and abroad.
One challenge researchers face is because no large-scale banks of tear samples exist, they have had to manually gather all their own samples, unlike blood samples, which can be purchased on large scales.
"Blood is already optimized," said Lindsay Rutherford, Ascendant's senior scientist. "Everything has been determined already: how to take it, how to process it."
Ascendant's scientists have been optimizing their own tear processes as they go.
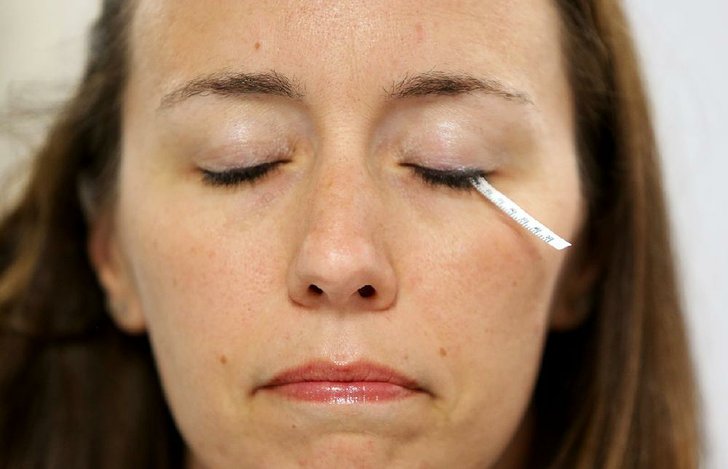
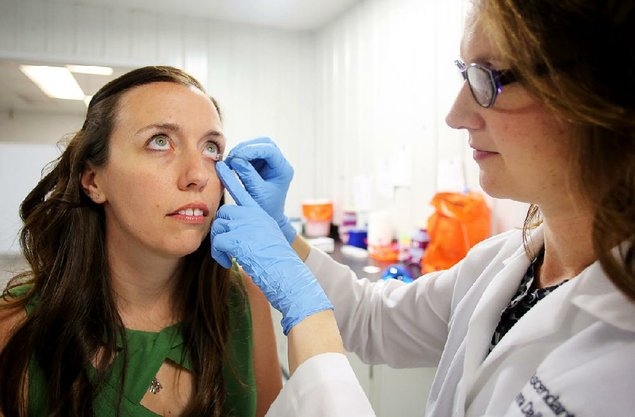
They use Schirmer's test strips to collect samples, which entails placing a small piece of filter paper under an eyelid for about two minutes. It irritates the eye enough to produce tears though has no lasting effects. They were originally developed by an ophthalmologist to measure tear production.
"It makes perfect sense," said Kevin Clark, CEO of NOW Diagnostics, a Springdale company that leases lab and office space to Ascendant.
"I had not personally thought about it before, but tears are part of the lymph system. Metastasizing tumors and everything show up in the lymph system, which is part of the immune system."
NOW produces tests that use a pinprick of blood to generate fast diagnostic results for a growing list of things, including pregnancy and infectious diseases. Ascendant is using NOW's cartridges to house its Melody test.
Clark said he has high hopes for other things that could be detected by Ascendant's technology. "Especially if we can put it in our platform, then you get a point of care device that gives patients real-time results," he said.
In the meantime, Ascendant has patent applications pending in the U.S. and abroad, and is in the process of raising a second round of funding, beyond the $2 million raised in 2013.
While tens of millions of dollars have been poured into the breast cancer cause, Moghadam said, "It just goes to research. You get nice papers, but where is the result? Where is the new drug? Where's the new diagnostic? Where's the new method? Where's the new medical device?"
He called this a "fundamental" problem in the industry, not just for breast cancer. Researchers are incentivized to publish papers, but there is a gap between those papers and translating them into products.
"This is research with a purpose for us," said Anna Daily, Ascendant's chief scientist, pointing out that in academia there is little quality control or verification of results.
By contrast, she explained, "When you're in a business or a company, you have to prove to your investors, the FDA and other regulatory organizations that we did what we said we did and it shows what we said it showed."
"They build buildings but in reality, nobody needs another $50 million building on a campus," Moghadam said.
"What you need is $50 million going into 10 companies, and if one of them is successful it can actually materially affect people with breast cancer."
Ultimately, he said, "If people really, truly want to cure cancer, there needs to be a balance between research and translating research into real products."
SundayMonday on 07/09/2017